Just Earth News | @justearthnews | 30 Jun 2021, 08:18 am Print
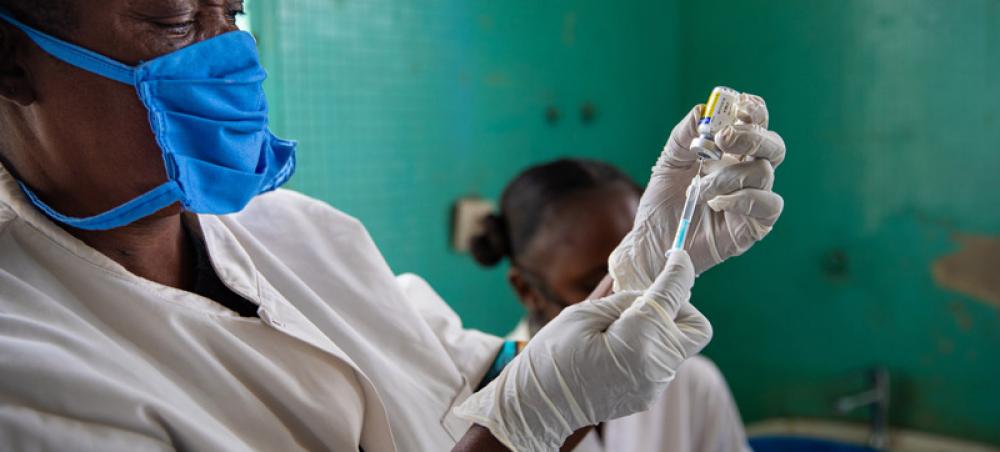 Covishield | Africa
Covishield | Africa Image credit: UNICEF/Sibylle Desjardins
The exclusion of India-made Covid-19 vaccine, Covishield, developed by AstraZeneca, from Europe's digital 'green pass' has become a cause of concern for the African countries with the African Union (AU) and Africa Centres for Disease Control and Prevention (Africa CDC) saying that the guidelines "risk the equitable treatment of persons" who were inoculated by vaccines from the EU-supported COVAX Facility, including the majority of the African Union (AU) Member States.
The European Union (EU) Digital Covid Certificate allows recipients of two doses of a Covid-19 vaccine approved by the European Medicines Agency (EMA), to travel freely within the bloc.
The pass recognises only AstraZeneca shots produced in Europe (branded Vaxzevria) and not the doses manufactured by the Serum Institute of India (SII), which is branded Covishield.
According to CNN, EMA has said that Vaxzevria is the only Covid-19 shot from AstraZeneca for which approval was requested.
"In the EU, the vaccine called Covishield does not currently have a marketing authorization. Even though it may use an analogous production technology to Vaxzevria, Covishield as such is not currently approved under EU rules," the EMA was quoted as saying.
"This is because vaccines are biological products," the agency stated. "Even tiny differences in the manufacturing conditions can result in differences in the final product, and EU law therefore requires the manufacturing sites and production process to be assessed and approved as part of the authorization process."
The EMA statement added: "Should we receive a marketing authorization application for Covishield or should any change to the approved manufacturing sites for Vaxzevria be approved, we would communicate about it."
The African Union noted in a statement that "..under such regulations, persons who received Covishield, despite being able to demonstrate proof of vaccination, would continue to be subject to public health restrictions, including limitations of movement and testing requirements, with considerable administrative and financial implications."
Since the expressed goal for the Serum Institute of India production is to serve India and lower-income countries, the SII may not apply for EU-wide market authorisation, meaning that the inequalities in access to “Green Passes” created by this approach would persist indefinitely, the AU pointed out.
The AU and Africa CDC also noted that AstraZeneca's Covishield vaccine was one of the first available candidates considered safe and efficacious through the World Health Organization's Emergency Use Listing process back in February.
SII CEO Adar Poonawalla said people who have been administered the Covishield shots are facing problems in travelling to EU countries.
"I assure everyone, I have taken this up at the highest levels and hope to resolve this matter soon, both with regulators and at a diplomatic level with countries," Poonawalla added.
An AstraZeneca spokesperson told CNN in a statement on Monday: "We are working closely with the EMA as they develop guidance to support opening of borders and relaxing restrictions, and this includes guidance on inclusion of Covishield as a recognised vaccine for immunisation passports."
- Abandoned at birth, Punch the macaque finds global love as crowds flock to Tokyo zoo
- YouTube Premium Lite just got a massive boost — Know all details
- Trump claims he stopped 35 million deaths by stopping India-Pakistan war
- Entrepreneur decides to shut down 16-year old eatery in London, cites harassment and Pakistani attacks
- Ubisoft bets big on Assassin’s Creed with strategic leadership revamp




-1763561110.jpg)
