Just Earth News | @justearthnews | 28 Jun 2018, 11:09 am Print
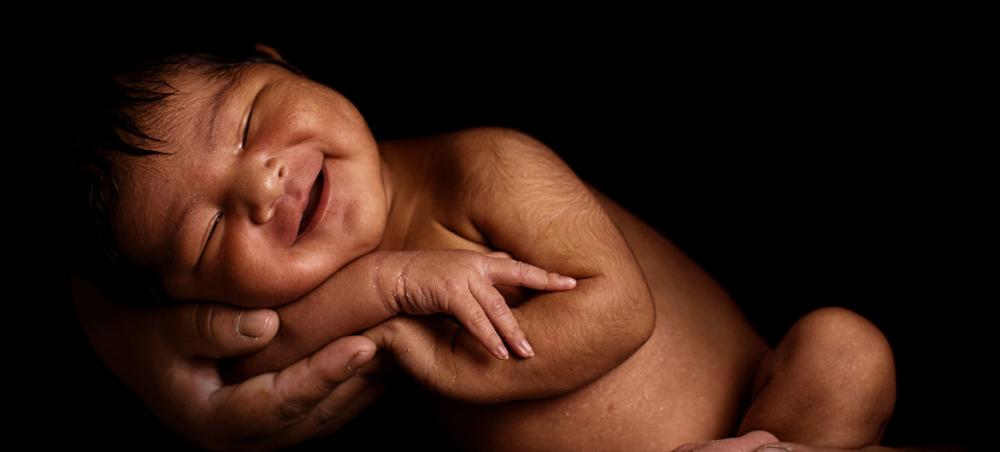
New York: A stand-by drug used to prevent potentially-fatal bleeding after childbirth has some new competition – with improved benefits, according a United Nations health agency-led report.
Excessive bleeding after childbirth still kills around 70,000 mothers a year and currently, Oxytocin is the first-choice medication, but it must be kept cold, unlike the new drug, Carbetocin.
The study, partly led, among others, by the World Health Organization (WHO) and published on Wednesday, suggests that the new drug which can be stored at normal temperatures, could save the lives of thousands in low- and lower-middle-income countries.
“This is a truly encouraging new development that can revolutionize our ability to keep mothers and babies alive,” said WHO Director-General Tedros Adhanom Ghebreyesus.
Since Oxytocin must be stored and transported at a cool two to eight degrees Celsius – a difficult task in many countries – numerous women lack access to the medicine. And if they can obtain it, heat exposure may render the drug less effective.
The study, published in the New England Journal of Medicine, has shown the heat-stable Carbetocin is not only as safe and effective as Oxytocin, but even without refrigeration – when stored at below 30 degrees Celsius and 75 per cent relative humidity – it retains its efficacy for at least three years.
Clinical trial
WHO notes that approximately 70,000 women die annually from postpartum haemorrhage – increasing the risk that their babies will also die within a month.
In the largest clinical trial of its kind, close to 30,000 women who gave birth vaginally were studied in Argentina, Egypt, India, Kenya, Nigeria, Singapore, South Africa, Thailand, Uganda and the United Kingdom.
Immediately after child birth, each woman was randomly injected with a single dose of either heat-stable Carbetocin or Oxytocin – revealing that both were equally effective at preventing excessive bleeding.
“The development of a drug to prevent postpartum hemorrhage that continues to remain effective in hot and humid conditions is very good news for the millions of women who give birth in parts of the world without access to reliable refrigeration,” said Metin Gülmezoglu of WHO’s Department of Reproductive Health and Research.
While Carbetocin has not yet been cleared for use beyond clinical trials, the next steps begin with a regulatory review, countries’ approval and then consideration by WHO’s Guideline Development Group.
However, the UN health agency said on Wednesday, that following the positive trial results, it will be working to advance affordable access to the potentially lifesaving drug in countries with a high maternal death rate.
UNICEF/Ilvy Njiokiktjien
- Alarming projection: Global breast cancer cases could cross 3.5 million by 2050, shows study
- Exam stress to emotional distress: Study reveals the dark side of academic pressure
- Vegetarian diet linked to lower risk of five major cancers, study finds
- Ukraine’s health system under fire: Attacks spike 20% in 2025, WHO warns
- A dog’s loving lick turned deadly — She woke up without her limbs




-1763561110.jpg)
